3D Intestine-on-chip model advances understanding of fungal infection
Together with colleagues from the Hans Knöll Institute and the Jena University Hospital, we have developed a near-physiological intestine-on-chip candidiasis model that simulates relevant pathogen-host interactions leading up to systemic candidiasis.

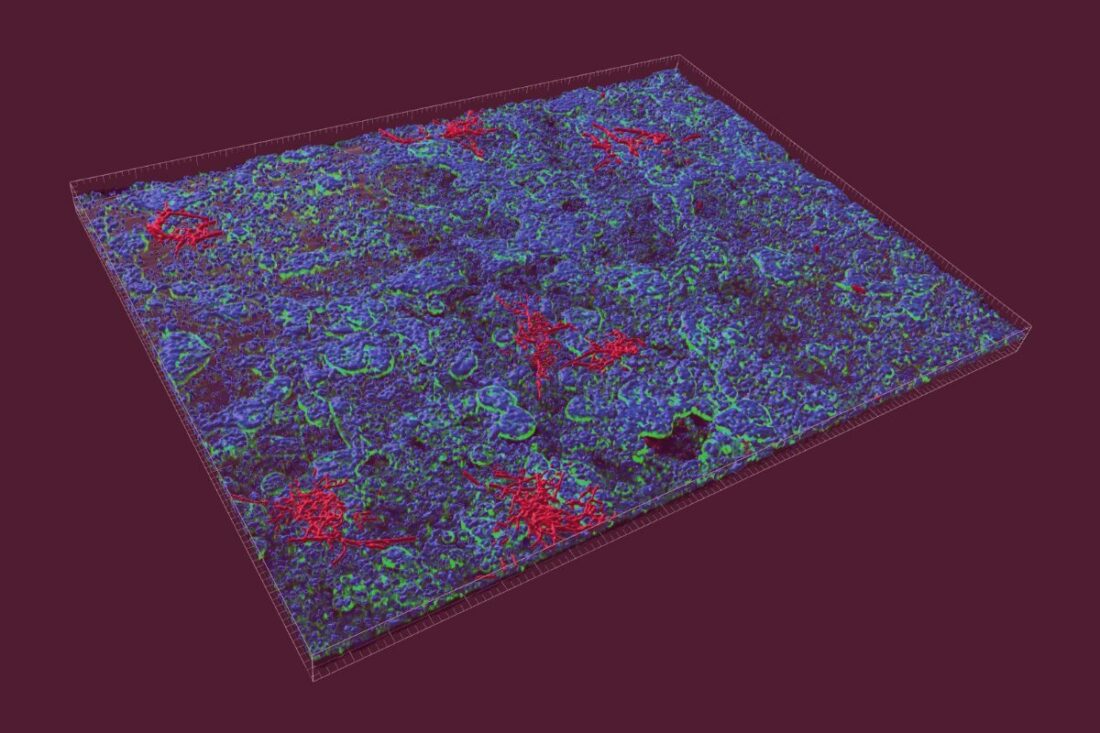
Fig. 1. 3D reconstruction of the intestinal epithelial cell infected with C. albicans (fluorescence image: C. albicans: red, F-actin: green, cell nuclei: blue; Copyright: Raquel Alonso-Roman, Parastoo Akbarimoghaddam)
Intestine-on-chip model for modeling fungal infections
In our recent publication published in the March issue of “Biomaterials” (T. Kaden et al., Modeling of intravenous caspofungin administration using an intestine-on-chip reveals altered Candida albicans microcolonies and pathogenicity, https://doi.org/10.1016/j.biomaterials.2024.122525) we have complemented microbiological techniques with advanced image analysis to gain a deeper understanding of the pathogenicity mechanisms of Candida albicans and the host responses to the fungus. Under certain conditions, C. albicans can invade and damage the intestinal tissue, enter the bloodstream, and disseminate to vital organs, causing a systemic disease. With approximately 1.5 million cases per year and a current mortality rate of around 64%, Candidiasis poses one of the most common nosocomial infections highlighting the importance for understanding the mechanisms of infection and treatment.
Organ-on-chip takes infection research to a new level
As a result of the successful collaboration between science, clinic, and industry our approach enables us to overcome existing limitations. For various reasons, animal models are not suitable for reproducing invasive candidiasis originating in the intestine. Differences regarding the immune system (J. Zschaler et al., 2014), (F. Hugenholtz et al., 2018), and metabolism (L. Demetrius 2005) make it difficult to transfer the results from laboratory animals such as mice to the conditions in humans. Existing in vitro models using monolayers of immortalized intestinal epithelial cells or co-cultures may have given valuable insights into various pathogenicity mechanisms, but they cannot fully mimic the host-pathogen-drug interactions observed in humans due to their limited complexity. In contrast, our organ model works entirely with human cells and tissues and reproduces the microanatomy of the intestine correctly. Thanks to the compartmentalization of the model, translocation and invasion can be studied accurately, and caspofungin administration via vascularity is also modelled exactly.
Learning more about host-pathogen interactions during fungal infection
Together with our collaboration partners we studied the growth and morphology of C. albicans microcolonies in the intestinal chip model after infection. Thereby, we analyzed the quantification of fungal burden, the processes of invasion and translocation as well as the impact on host tissue integrity. An image-based analysis of the morphometry was also performed. As a result, we observed how C. albicans attaches to and invades the epithelium, partially translocated in the vasculature and disrupts tissue integrity. The latter was manifested by dysfunction of cell-cell contacts in close contact to microcolonies and reduced cell nuclei (cell death) and a high amount of microcolonies.
Making our proven model even better
We have optimized our existing intestine-on-chip model (described in M. Maurer et al., 2019) with the following steps:
- formation of a 3D intestinal epithelial tissue architecture with improved barrier function, induced by perfusion in the chip model
- integration of tissue-resident macrophages as immune cells to investigate inflammatory reactions
- incorporation of vascularity enabling the investigation of microbial translocation into this “bloodstream”-like compartment and mimicking intravenous application of antifungal drugs via the vascular perfusion.

Fig. 2. Conceptualization of the intestine-on-chip candidiasis model. (A) Schematic cross-sectional representation of human intestinal tissue within the biochip. (B) Representative photographs of intestine-on-chip models with attached microfluidic reservoirs (vascular: yellow/pink medium; intestinal: orange medium) under bidirectional circular perfusion (top image). (C) Overview of tissue processing prior to image-based analysis 12 hpi and microbiological read-outs 24 hpi. Parts of this figure were created with BioRender.com. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of the article.). © The Authors. Published by Elsevier Ltd.
Overcoming treatment failure and antifungal drug resistances
The initial treatment of invasive candidiasis typically involves echinocandins, such as caspofungin. As B.J. Kullberg et al. showed 2011 and G.D. Brown et al. described 2012, treatment failure is often observed in patients due to development of antifungal resistances and varying drug metabolism, contributing to the devastating mortality rate of invasive candidiasis.
With our model, we can now investigate precisely, how effective antifungal treatment is in both wild-type and echinocandin-resistant strains of C. albicans and how it affects pathogen-host interactions in situ. Our experiments highlight that caspofungin reduces C. albicans invasiveness, fungal burden, intestinal barrier disruption, host immune response, and alters microcolony morphology in a wild-type, but not in the echinocandin-resistant strain.
Streamlining microbiological and quantitative image-based methods for enhanced prediction
In summary, our organ-on-chip model is a powerful tool for understanding host-pathogen interactions and the impact of antimicrobial drug treatments. Particularly the combination of microbiological and image-based analyses allows an accurate evaluation of both the severity of the infection and the efficacy of antifungal drugs, such as caspofungin, and thus significantly advances our understanding of invasive candidiasis.
More interesting articles:
Blog
Organ-on-chip applications by organ type – what has been done?
This blog lists examples of organ-on-chip models by organ type that have been used in the past, providing the respective literature for your reference.
Read MoreBlog
Exploring infectious disease dynamics through organ-on-chip technology
This blog explores established infection models using our organ-on-chip technology and their implications for scientific research.
Read MoreBlog
Immunocompetent Organ Models – the Future of Biomedical Research
One crucial factor that plays a pivotal role in the success of organ-on-cip models is immunocompetence. In this blog post, we delve into the significance of immunocompetence in organ-on-chip models and how it opens new avenues for advancing medical research.
Read More