Liver-on-a-chip – Revolutionizing assessment of drug-induced liver injury
The process of drug development is an intricate journey that requires thorough investigation to ensure the safety and efficacy of newly developed pharmaceutical compounds. One of the critical aspects of this process is assessing the potential adverse effects of drugs on the liver, a vital organ responsible for metabolization and detoxification in the body.
We recently published in Nature Scientific Reports titled “Evaluation of drug-induced liver toxicity of trovafloxacin and levofloxacin in a human microphysiological liver model”. This case study sheds light on the effects of the known hepatotoxic antibiotic trovafloxacin and the commonly used analog levofloxacin on human liver tissue, using an innovative chip-based liver model. Trovafloxacin did not induce liver injury in animal models and in preliminary in vitro studies but was withdrawn from the market after serious hepatic adverse effects in clinical patients, whereas levofloxacin is approved and commonly prescribed. We therefore wanted to evaluate whether our in vitro liver model is superior in identifying these adverse effects by trovafloxacin and validate the non-toxic profile of levofloxacin.
Summary of the most interesting findings:
- We advanced a previously published microphysiological model of the human liver (Rennert et al. 2015) suitable for drug testing by including liver sinusoidal endothelial cells and resident immune cells combined with HepaRG hepatocytes by Biopredic
- The model enables “bloodstream-like” intravenous drug application via the vascular perfusion
- Trovafloxacin induced severe acute liver toxicity in the model at human-relevant concentrations
- Trovafloxacin toxicity was associated with:
- decreased cell viability
- elevated liver injury markers lactate dehydrogenase (LDH) and alanine aminotransferase (ALT)
- enhanced pro-inflammatory cytokine release within the vascular perfusion
- generation of reactive oxygen species and depletion of glutathione in hepatocytes
- In comparison to previous models, no additional inflammatory stimulus was required to induce liver injury suggesting an intrinsic toxicity induced by trovafloxacin
- The structural analog levofloxacin did not induce comparable toxicity and was generally safe in the liver model even at high concentrations
- The liver model was advantageous compared to two-dimensional monocultures of endothelial cells and hepatocytes in detecting trovafloxacin toxicity
For a more detailed description of the work, keep reading
Conventionally, drug testing has relied on animal models, but the differences between human and animal physiology can sometimes lead to misleading results. On the other hand, simply two-dimensional cell culture models neglect the complexity of a whole human organ. To closely mimic the human liver microanatomy, to emulate the correct drug administration route and to support convenient dissection of individual cell compartments, we established a three-dimensional and perfused microphysiological model of the human liver within our Dynamic42 biochip platform.
This three-dimensional model consists of a vascular, perfused compartment with liver sinusoidal endothelial cells and resident macrophages and a hepatic compartment with HepaRG hepatocytes (Biopredic). HepaRG cells were used as an adequate alternative to human primary hepatocytes, the “gold standard” for in vitro drug testing, due to their availability, comparable CYP enzyme expression and presence of relevant efflux and uptake transporters.
The vascular and the hepatic compartment are separated by a porous membrane, on which the cell types are seeded and cultured independently. Subsequently, the test substances trovafloxacin or levofloxacin were applied in different human-relevant concentrations via vascular perfusion for up to 7 days.
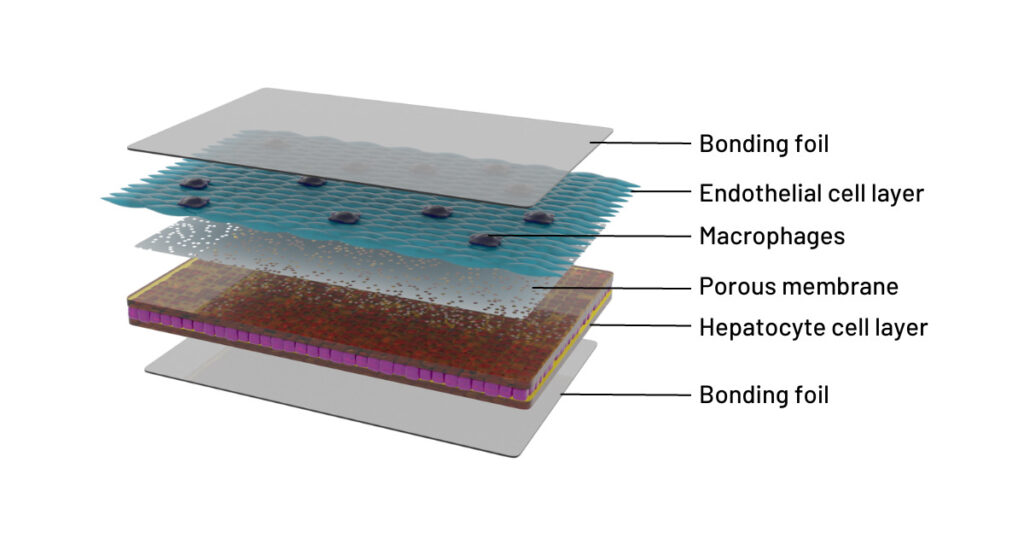
Cross-sectional illustration of the 3D liver model in the Dynamic42 biochip. The model consists of an endothelial cell layer (turquoise) co-cultured with resident macrophages (indigo) and an opposing hepatocyte cell layer (purple/brown). Cell layers are separated by a porous membrane (grey, with pores). The distance between cell layers and membrane is artificial for clearer representation of the model. The cultivation chambers are tightly sealed at the top and bottom with a bonding foil (grey, uniform).
The application of trovafloxacin at clinical-relevant concentrations resulted in acute liver tissue damage within the first three days. This was demonstrated by an increased release of the clinical liver injury markers lactate dehydrogenase (LDH) and alanine aminotransferase (ALT) in the vascular perfusion and an enhanced release of inflammatory cytokines. After seven days, a reduction in overall tissue viability and number of viable cells in both vascular and hepatic compartments were detected. While treatment of statically cultured monocultures of liver sinusoidal endothelial cells showed no indication of toxicity at equal concentrations of trovafloxacin, damage to liver sinusoidal endothelial cells was also observed in the liver model due to the spatial separation of the cell types. Whereas adverse drug reactions are often missed in static in vitro monocultures, the multicellularity and spatial separation of the different cell compartments (for the liver sinusoid: parenchymal= hepatic, non-parenchymal=vascular) enables the dissection of contributing cell types and consequently more accurate results for toxicity testing. Furthermore, we identified increased generation of reactive oxygen species with concomitant depletion of protective glutathione in hepatocytes as one of the suggested mechanisms of trovafloxacin toxicity. These deleterious effects have not been shown for levofloxacin at the same concentrations and implicate a non-toxic profile of the drug.
Previous studies have demonstrated similar effects using advanced in vitro liver models, however, application of supraphysiological drug concentrations, inclusion of non-native cell types, lack of immune cells or non-physiological drug administration are limiting constraints. Moreover, in most of these models, the toxic effect of trovafloxacin could only be induced by an inflammatory co-stimulus. Our liver model allowed the detection of trovafloxacin-induced toxicity without an additional immunostimulant reflecting more physiological conditions.
Implications and Future directions
The findings contribute to the understanding of trovafloxacin toxicity and verified the non-toxic profile of the approved drug levofloxacin. Therefore, we emphasize the potential of the human liver microphysiological model as an in vitro platform for the evaluation of drug-induced liver injury. The model could be relevant to address limitations of conventional animal models and two-dimensional cell cultures, and could prove useful to detect undesirable drug adverse before human clinical trials.
In the future, we aim to validate our system with additional reference compounds and drugs with unknown effects to minimize the risk of liver injury in patients already in the preclinical phase.
Further reading
Read our recent liver-on-chip publications from us and our collaborators:
Rennert et al. 2015: A microfluidically perfused three dimensional human liver model
More interesting articles:
Blog
Organ-on-chip applications by organ type – what has been done?
This blog lists examples of organ-on-chip models by organ type that have been used in the past, providing the respective literature for your reference.
Read MoreBlog
Exploring infectious disease dynamics through organ-on-chip technology
This blog explores established infection models using our organ-on-chip technology and their implications for scientific research.
Read MoreBlog
Immunocompetent Organ Models – the Future of Biomedical Research
One crucial factor that plays a pivotal role in the success of organ-on-cip models is immunocompetence. In this blog post, we delve into the significance of immunocompetence in organ-on-chip models and how it opens new avenues for advancing medical research.
Read More

