Introduction to drug discovery and drug development
Bringing a drug from bench to bedside is an intricate process that requires drug development, clinical trials, and regulatory approval. The process is designed so that innovative new medicines are provided in the most effective and safe way possible to the patient. In this blog, we will provide you with an introduction to the drug development process from target validation to post-market surveillance to give you a better understanding on how new drugs are being developed.
What is drug discovery?
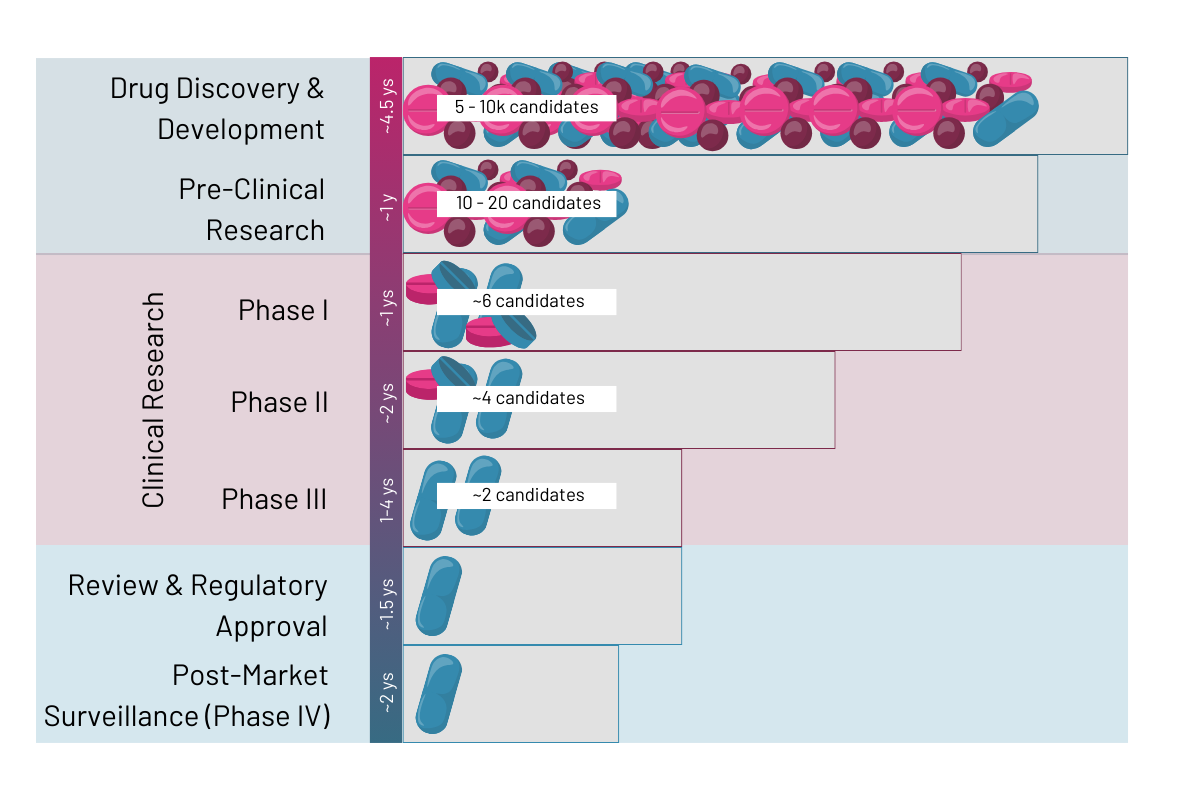
Drug discovery is the process leading from target discovery up to the regulatory approval of a new drug. It involves drug discovery and development, pre-clinical research, clinical research as well as regulatory review and post-market surveillance (Fig. 1). The drug development process is an extensive and expensive process that can take on average 10 to 15 years from target research to final drug on the market and amounts to around 0.7 to 2.7 billion US dollars (Rousseaux 2023 et al.). Typically, only 1 out of 5,000 – 10,000 potential drugs from drug discovery will reach approval by the health authorities (Rousseaux 2023 et al.). According to Sun 2022 et al. the 90% of drugs failing after clinical trial phase 1 are due to lack of clinical efficacy (40%–50%), unmanageable toxicity (30%), poor drug-like properties (10%–15%), and lack of commercial needs and poor strategic planning (10%).
The stages of the drug development process:
Stage 1: Drug discovery and development
Stage 2: Pre-clinical research
Stage 3: Clinical research
Stage 4: Review and regulatory approval
Stage 5: Post-market surveillance
Stage 1: Drug discovery and development – Science is the foundation

What is a target?
The first step in creating new drugs is always the identification of a new target that has the potential to treat or prevent a disease. Typically, such targets are proteins associated with a disease or condition in the human body or are targets on the pathogen that causes the disease itself.
Targets are identified through in-depth analysis of the underlying disease mechanisms or pathogenesis of a disease. Once understood, the researchers can pin down proteins that are vital to the pathways and might be suitable targets for drug discovery.
Screening and identification of hits
The identified targets are subjected to either high-throughput, fragment-based or virtual screening of compounds to identify molecules that can interact with them and modulate their behaviors (Domainex). At this stage, very large quantities of molecules are screened against the target to identify what is also called a “hit”. A hit is a compound that interacts with the target and results in the required therapeutic effect. There are several strategies to identify such hits, but they all must ensure to reduce the number of false positives and at the same time provide enough initial hits for further screening.
Lead identification and optimization
This stage is also called “hit-to-lead” as it aims to identify leads among the fast pool of hits obtained in an initial screening phase that can be taken through to the drug discovery stages. The essential aim of this stage is to further narrow down the number of potential drug targets into a smaller subset that shows biological activity against the target and has desirable properties for drug development. A selection occurs by properties such as solubility, permeability, protein binding, metabolic stability, and in vivo pharmacokinetics (Sun 2022 et al.). The process to optimize and select potential leads involves systematic modification of the chemical structure of the hits to enhance biological activity, improve selectivity and enhance pharmacokinetic properties involving chemical design, synthesis, and testing.
Besides testing for the activity of the compounds toward the target, this phase also selects hits by factors such as their solubility, metabolic stability, and the potential for large scale synthesis. This selection occurs as potential drugs do not need to be just safe and effective in the human body but also practical for production in large quantities.
Once leads are selected, they proceed to the pre-clinical research stage, a phase dedicated to safety and efficacy testing, and thereby laying the groundwork for the clinical stages.
Stage 2: Pre-clinical research – The 3R’s
The main aim of this phase is to identify drugs that could pose toxic to the human body and determine an ideal starting dosage before they are brought into clinical trials. The assessment of toxicity and thereby identification of any potential adverse effects to the human body is done via in vitro and in vivo methods. Should the drug show signs of toxicity to the model system, it would be taken out of the trial and not be considered for further studies.
Toxicity and dosage are determined via pharmacokinetics, pharmacodynamics and toxicology testing which are further explained below (Vugmeyster 2021 et al.).
/ Pharmacokinetics – is to understand the potential adsorption, distribution, metabolism, excretion (ADME) of a compound in the human body.
/ Pharmacodynamics – is about determining the biochemical and physiological effect the compound could have on an active site in the human body relative to the concentration of the drug.
/ Toxicology testing – helps to determine to which degree the compound negatively impacts the human body and its organs.
Typically, this stage of drug development is shorter than the clinical phases, however the studies must follow good laboratory practice (GLP) and provide exhaustive information on dosing and toxicity of compounds.
In vivo vs. in vitro models
Lead compounds must be tested on systems replicating living conditions and human cells. Typically, testing would start on in vitro models and then move to in vivo. The main difference being that in vitro systems are artificial but typically using human cells and that in vivo systems are living model organisms but of animal origin.
In vitro models offer the significant advantage that compounds can be tested on human cells and in that they provide a more controlled and standardized environment.
Here are some examples of in vitro models that are used in preclinical testing:
/ 2D cell culture
/ 3D cell culture
/ Organoids or Spheroids
/ Organs-on-chip
Depending on the type of in vitro system they can be run in larger badges or high throughput, but typically those systems are less complex and can only give a first indication towards the safety of the drug.
In contrast, more complex in vitro systems such as organs-on-chip offer the unique advantage of using human (often primary) cells and an intricate assembly of the cells to a physiological tissue representing the minimal viable part of a human organ. Organs-on-chip offer a number of unique advantages:
/ Simulation of mechanical stress to cells impacting several signaling pathways
/ Vascularization to mimic blood flow
/ Introduction of immune cells to mimic the immune response to the drug in the human body
/ Interaction of microorganisms with human tissue
/ Physiological assembly and formation of cellular tissue through perfusion
/ Specific cell types or tissues can be studied
/ Tissue exposure and selectivity in disease and normal tissues can be observed
/ You can read more on this topic here
Animal models on the other hand often greatly differ from humans but can give a more complex idea on how drugs a metabolized in a complex body with multiple organs.
That said modern organ-on-chip models offer the opportunity to create multi-organ-chips. Enabling the researcher to test for the metabolization of their drugs under more natural conditions giving a better idea on the dosage needed in the clinic.
Many critical experiments such as understanding liver toxicity of the compounds can be tested using models such as a liver-on-chip giving early clues to the adverse effects of compounds on human organs (Kaden 2023 et al.).

Why is it important to reduce animal testing?
Often animals such as mice are still the chosen model when it comes to testing at this stage. However, animal models are expensive and very laborious to maintain and often results obtained in animals are not reproducible in the human body leading to high failure rates in clinical trials.
Much more cost-effective and physiological are organ-on-chip models created from human cell lines, primary cells or stem cells. They enable the testing of possible drug candidates on human physiological systems without the need for animal models. Additionally, they enable direct assessment of tissue exposure and selectivity by the drug, allowing for more precise determination of dosage and drug specificity and potency (Sun 2022 et al.).
Fortunately, there is a movement in the pharma industry towards the 3R principle of refining, reducing, and replacing the use of animals in research. This movement has also reached the regulatory authorities who subsequently reduce the need for animal testing as a prerequisite for drug approval (Science)
Stage 3: Clinical research – From lab to pharmacy

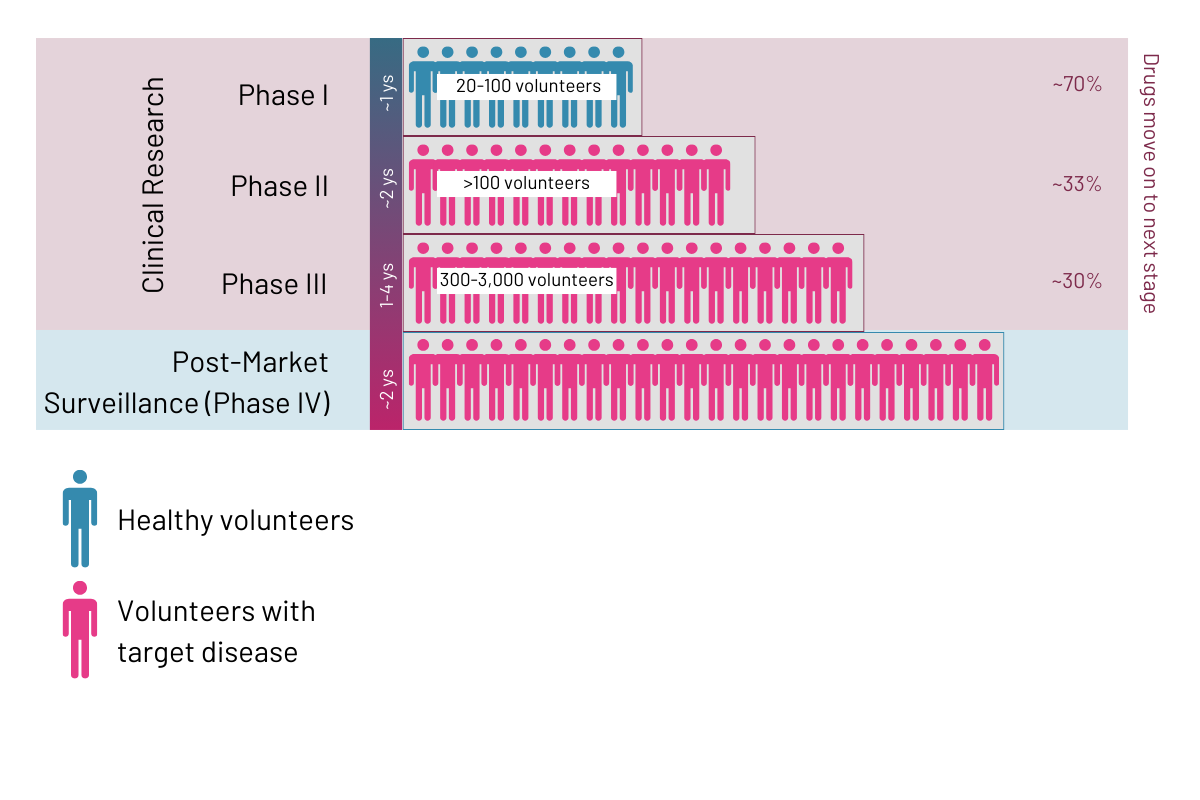
The clinical drug development stage consists of four phases of which each is dedicated to test the drug’s effectiveness and safety in the human body (FDA). In the first phase healthy volunteers are subjected to the drug to evaluate the safety and dosage of the compound. In phase two the drug is given to a small group of patients. The goal of this phase is to understand efficacy and optimal dose of the compound and evaluate any potential side effects to the patient. To receive early indication on whether the drugs affect targets in the intended way, pharma companies will sometime conduct proof of concept studies which are a mix of phase one and two, in which the drug is given to only a small group of very well-defined patients. Phases three and four are the largest clinical phases and involve testing the drug on several thousands of patients. Again, effectiveness and side effect of the drug are monitored, and comparisons are drawn to preexisting treatment options.
Phase 1
This study is typically conducted with a lower number of healthy volunteers (20 -100) or patients with the disease or condition and can take several months. According to the FDA, about 70% of drugs typically move on to the next phase (FDA). The purpose of this stage is to assess safety and dosage of the drug.
Phase 2
This is the first clinical phase in which people with the targeted disease or condition are required, and typically several hundred of test subjects are used in this phase. The phase can take from several months up to 2 years and about 33% of drugs move on to the next stage (FDA). The purpose of this phase is to determine efficacy and side effect of the compounds.
Phase 3
This phase involved a larger subset of patients (300 to 3,000) which have the targeted disease or condition. It is also a very long phase, taking between 1 and 4 years, and according to the FDA only about 25 to 30% of drugs move on from the third phase to the fourth (FDA). The aim of this stage is to monitor efficacy and adverse reactions to the drug.
Phase 4 (post-market surveillance)
This phase occurs after the drug has been approved by the health authorities and is also referred to as post-market surveillance phase. This means that the drug is available on prescription to the general public but is still being monitored for any potential side effects and issues that might arise. Learn more under “Step 5”.
Stage 4: Review and regulatory approval

Once clinical trials are completed, and the drug has been deemed safe and effective by its producer, it needs to be authorized by the local health authorities. Depending on the country in which the drug will be brough to market, there are different authorities to be considered, here are just a few examples:
/ United States – U.S. Food and Drug Administration (FDA)
/ Canada – Health Canada
/ China – The National Medical Products Administration (NMPA)
/ India – The Central Drugs Standard Control Organization (CDSCO)
/ Europe – European Medicines Agency (EMA)
/ Germany – Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte, BfArM) or Paul-Ehrlich Institut (PEI)
/ United Kingdom – Medicines and Healthcare Products Regulatory Agency
/ For a full list of health care authorities take a look here
Depending on where in Europe a drug will be brought to market there are three different routes that can be taken (IQWIG):
/ The National procedure: The documentation is only sent to the local authorities and the drug will only be marketed in that one specific country. In Germany depending on the type of drug it is either send for approval to the BfArM or PEI.
/ The decentralized procedure: The aim is to market the drug only in some but not all EU countries. If the drug has been previously approved in one of the countries in the European Economic Area (EEA) Mutual Recognition Procedure (MRP) applies in which the authorities can grand permission based on the grand approval of the other country.
/ The centralized procedure: This route applies either if the target is to market the drug in the EEA or if the drug applies to a specific class of compounds (f.ex. drugs against cancer or autoimmune diseases). In this case the documentation is to be sent to the EMA for evaluation.
Those authorities will check all documentation compiled from pre-clinical as well as clinical trials supporting the final intended use of the compound. Health authorities will evaluate drugs according to their pharmaceutical quality, safety, therapeutic efficacy, and tolerability by the human body. The exact definition and criteria for a compound to be considered save depends highly on the local health authority and might differ greatly between countries. In Germany, after approval the drug is initial on the market for 5 years and can then apply for extension to receive unlimited approval if there are no safety concerns.
Step 5: Post-Market surveillance – Clinical phase 4

Once the drug is approved by the authorities and launched in the market, drug safety monitoring will continue to ensure efficacy and safety of the drug in the wider market. The most important factor to be considered hereby is the risk-benefit factor. Should this change and the risks start to outweigh the benefits of a compound, a recently approved drug could be taken off market. This additional stage is necessary as clinical research is comprehensive and thorough, but could never provide the full picture like a drug that is already in the market for some years and has been taken by thousands of patients. Depending on the issues that are being reported in the post-market phase additional cautions, dosage information or more serious measures, such as taking the product off market, can be taken by health authorities.
Conclusion
The drug development pathway is designed so that only safe and effective drugs make it to market. The whole process can take several years, is very costly, and only very small amounts of drug candidates will make it into the final clinical stages. Especially when looking at failure rates in early clinical trials, there is room for improvement by utilizing more physiological models in the pre-clinical trial phase to detect human toxic compounds early on and determine optimal dosage for clinical trials. Once clinical trials are completed, documentation will be sent to and reviewed by the local authorities which assesses pharmaceutical quality, safety, therapeutic efficacy, and tolerability of the compounds. Once approved and on the market, the extended post-market surveillance phase will determine if, even when administered to thousands of patients, the benefits of the new drug indeed outweigh the risks of potential side effects.
More interesting articles:
Blog
Organ-on-chip applications by organ type – what has been done?
This blog lists examples of organ-on-chip models by organ type that have been used in the past, providing the respective literature for your reference.
Read MoreBlog
Exploring infectious disease dynamics through organ-on-chip technology
This blog explores established infection models using our organ-on-chip technology and their implications for scientific research.
Read MoreBlog
Immunocompetent Organ Models – the Future of Biomedical Research
One crucial factor that plays a pivotal role in the success of organ-on-cip models is immunocompetence. In this blog post, we delve into the significance of immunocompetence in organ-on-chip models and how it opens new avenues for advancing medical research.
Read More

